|
Part Two, The Mighty Atom Revisited:
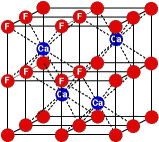
F wants another electron, so it can get a negative one (-) charge. Ca easily loses two electrons, getting a plus two (2+) charge. The two ions (Ca and F) attract each other and form the chemical compound calcium fluoride or CaF2 (one calcium ion bonds to two fluorine ions). In the crystal growth process, a square (cubic) three-dimensional structure of alternating Ca and F spheres grows into the mineral fluorite. The cubic structure is the geometric pattern for the crystal growth for the mineral fluorite. 4 Definitions: A
mineral is defined as a naturally occurring
(no artificial help from man), homogeneous
(all the same) solid
(not a liquid or a gas) with a
definite (but generally not fixed, or just one type) chemical composition (CaF2) and a highly ordered atomic arrangement (cubic structure).
Minerals usually (but
not always) are formed by
inorganic (not organic) processes1.
An organic process involves formerly living things, like the
formation of petroleum hydrocarbons by heat and pressure working on
buried plant (carbon) matter. An
inorganic process could be a hydrothermal (hot water) fluid that keeps
the ions moving too fast to combine (grow), then cools enough to allow
the crystal growth of a mineral. Crystals are a polyhedral form (geometric solid), with a specific set of faces, edges, and corners (cubes have six
faces or sides, eight edges, and eight corners), which is consistent with the geometric packing of the atoms within the
crystal (the geometric form of a cube, which the atoms create as
they crystallize into a mineral). 2
Sometimes
a crystal may not visually appear to have a geometric form that is
typical for that mineral. The
crystal form may be present at the microscopic level, but is invisible
to the human eye. The
crystal represents a pseudomorph,
a mineral whose outward crystal form is that of another mineral3.
Crystals of one mineral can melt or dissolve by geologic or
geochemical processes, and leave their crystal pattern as an open
space (void) without removing different minerals or rocks surrounding
the missing mineral. Liquid
fluorine and calcium ions later enter the void left by the missing
mineral, and re-crystallize in the missing crystal shape, while
maintaining a cubic structure invisible to the eye.
Other
times the crystal form can be the result of several related basic
crystal forms combining (cubic is one of seven simple crystalline
forms which can combine in the isometric system). Jim Barton Thanks again to my professors at the University of Nebraska at Omaha, my college mineralogy text (Manual of Mineralogy, Klein and Hurlbut, 20th Edition)1; Simon & Schuster's Guide to Rocks and Minerals2; Dictionary of Geological Terms, 3rd Edition, Bates and Jackson, AGI3; Mineralogy, Sinkankas; and my college chemistry text (Chemistry, the Central Science, 4th Edition, Brown & LeMay) for providing the basis for this article. Continuation
of this Series of Articles: |